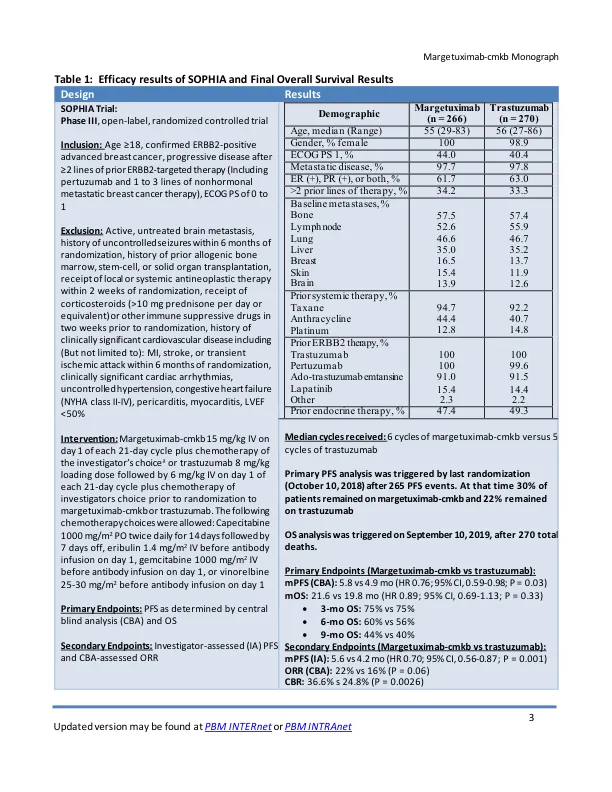
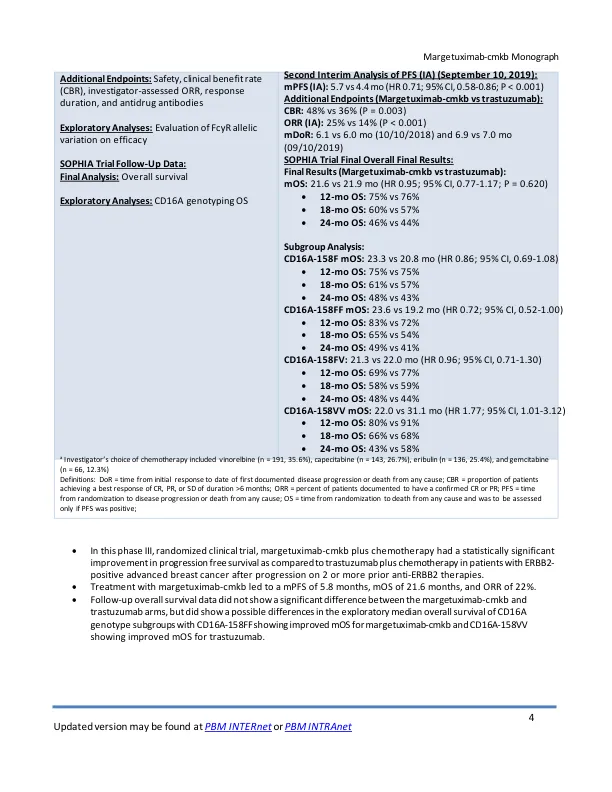
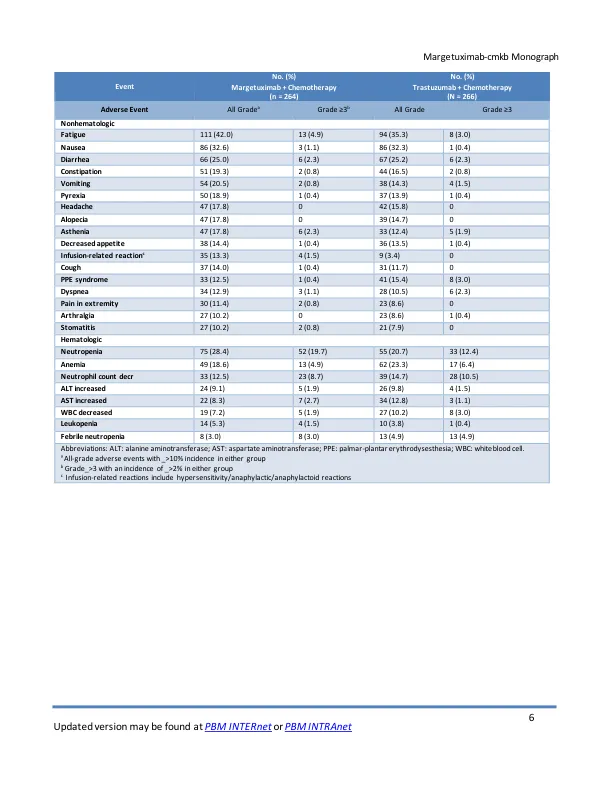
• The safety of margetuximab-cmkb was evaluated in the SOPHIA trial, a phase III trial of patients with ERBB2- positive advanced breast cancer in combination with chemotherapy when compared to trastuzumab and chemotherapy • Common adverse events (≥20% of patients; margetuximab vs. trastuzumab) included fatigue (42% vs 35.3%), nausea (32.6% vs. 32.3%),腹泻(25%vs 25.2%)和中性粒细胞减少症(28.4%vs 20.7%)和呕吐(20.5%vs 14.3%)•最常见的3级或更高的事件或更高的事件(≥1%; margetuximab vs trastuzumab)(包括trastuzumab)包括中性含量为19.7%vs 12.7%vs n.4%vs vs n.4%vs vs vs vs n.4%vs vs vs vs vs n.4%vs vs vs vs vs n.4%vs vs vs n.4%vs vs vis vis vs n vers courtiane countialia 10.5%),贫血(4.9%vs 6.4%),疲劳(4.9%vs 3.0%),AST增加(2.7%vs 1.1%),ALT增加(1.9%vs 1.5%),WBC降低(1.9%vs 3.0%),Leukopenia(5.3%)(5.3%),NAUSEA(1.4%)(0.4%vs vs vs vs vs vs vs vs vs.4%vs vs vs vs vs nausea vs vs vs.4%vs vs vs。 2.3%), asthenia (2.3% vs 1.9%), infusion-related reaction (1.5% vs 0), dyspnea (1.1% vs 2.3%), abdominal pain (1.5% vs 1.1%), hypokalemia (1.5% vs 0.8%), hypertension (1.9% vs 0.8%), pneumonia (1.9% vs 2.6%), and syncope (1.5%vs 0)
margetuximab-cmkb(Margenza)国家药物专着2023年12月
主要关键词