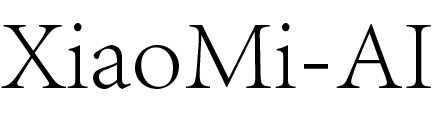机构名称:
¥ 5.0
1. Regulatory category .... 41 2. Validity period .... 41 3. Storage method in packaged condition .... 41 4. Handling precautions ..... 41 5. Materials for patients .... 41 6. Same ingredients and efficacy drugs .... 41 7. International birth date ..... 41 8. Date of manufacture and sales approval and approval number, drug price standard Date of listing, date of sale start date ... 42 9. Date and contents of the addition of efficacy or效果,额外使用和剂量更改等。...42 10。重新检查结果,重新评估结果及其内容的发布日期.... 42 11.重新检查期.... 42 12.有关药物限制的信息.... 42 13. 42 13.各种代码.. 42 14。
制药访谈表