• First Australian Patient Dosed in Phase 1b Azer-cel Clinical Trial, Targeting Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL): Imugene Limited has announced that the first Australian patient has been dosed in the Phase 1b clinical trial of azer-cel (azercabtagene zapreleucel) at the Royal Prince Alfred Hospital (RPAH) in Sydney. Azer-cel is an allogeneic, off-the-shelf CAR T-cell therapy designed to shorten treatment timelines and expand accessibility for patients who have limited options, particularly those with challenging, aggressive forms of DLBCL. This announcement follows promising data from the Company's U.S. trial sites, where three patients achieved complete responses (CR) despite having failed multiple prior treatments. In Cohort B, which includes lymphodepletion chemotherapy and interleukin-2 (IL-2), responses have extended beyond 90 and 120 days, indicating robust signs of durability. The dosing of the first Australian patient represents an important step in assessing the therapy's broader clinical applicability and potential benefits for patients with advanced lymphoma.
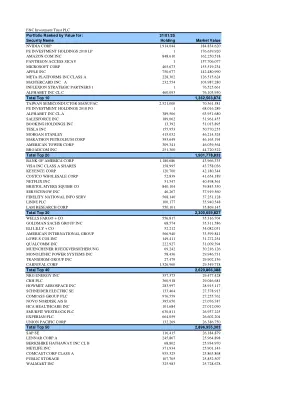
Investment Highlights
主要关键词