机构名称:
¥ 1.0
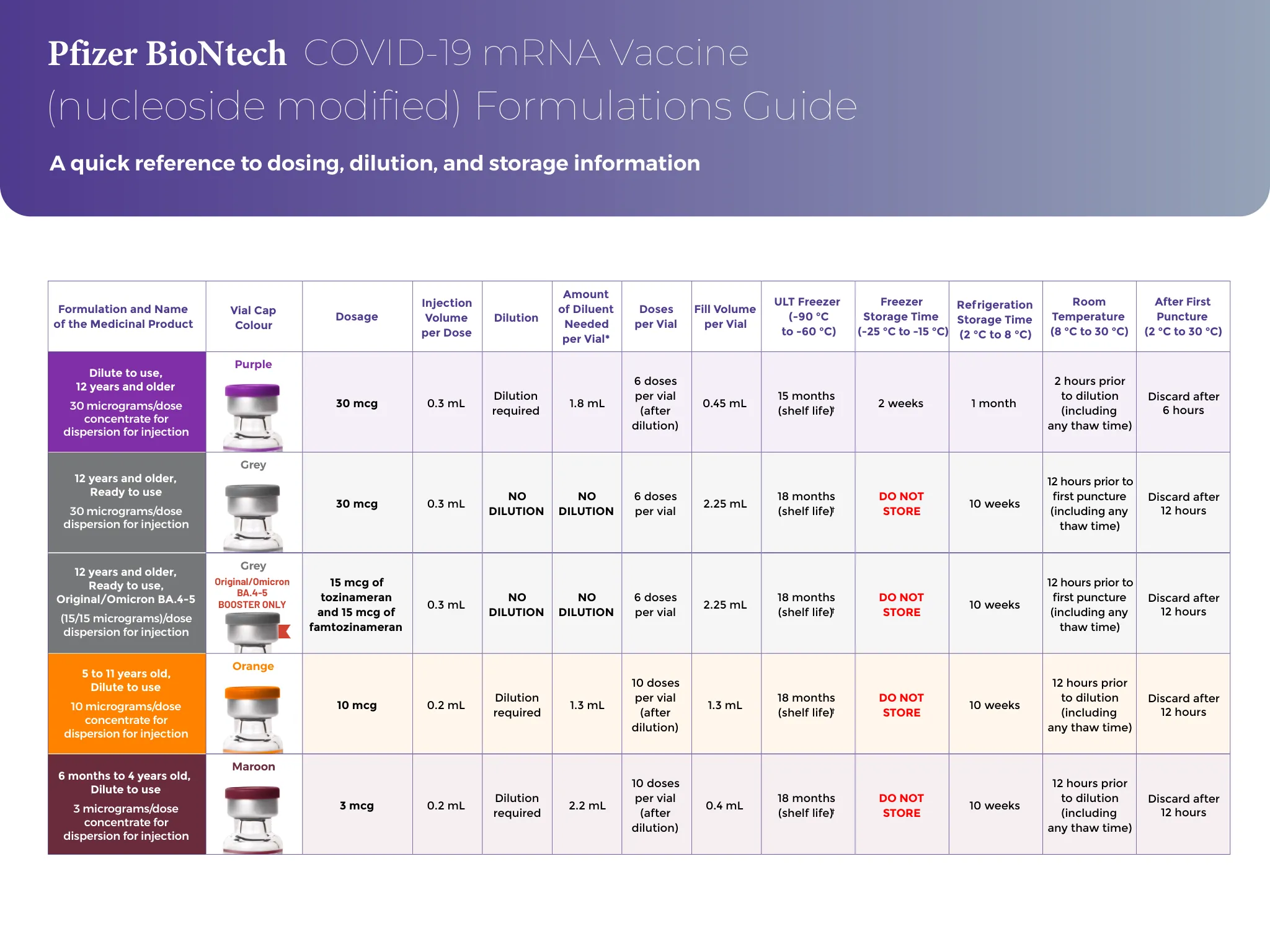
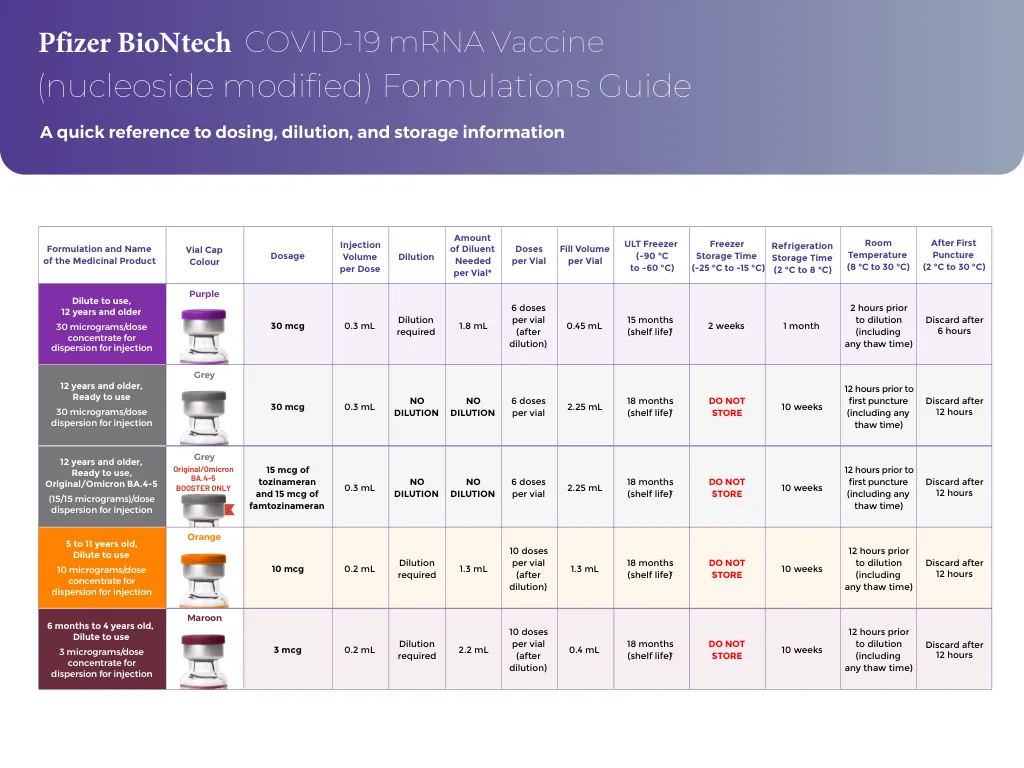
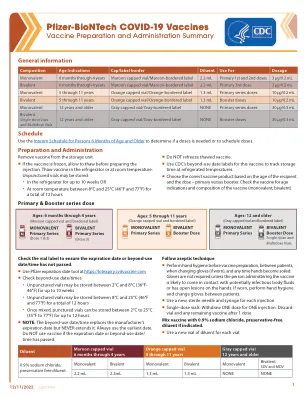
A Marketing Authorisation has been granted in the EU: • for the product COMIRNATY COVID-19 mRNA Vaccine (nucleoside modified) (Pfizer-BioNTech COVID-19 Vaccine in several countries) for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in infants and children aged 6 months to 4 years of age (3 micrograms/dose), in children aged 5 to 11 years (10微克/剂量)和12岁及以上的个人(30微克/剂量)•对于comirnaty原始/Omicron Ba.1和Comirnaty原始/Omicron BA.4-5 Covid-19 covid-19 mRNA疫苗(核苷)(Nucleoside修饰)的活性免疫,以防止COVID-COVID-COVID-19的较旧的人(15岁)(SARS-COV-2),[15/sys Cov-2(15/)(sars-cov-2)(( Micrograms)/剂量],以前至少接受过针对COVID-19的初级疫苗接种课程
Pfizer Biontech Covid-19 mRNA疫苗