机构名称:
¥ 3.0
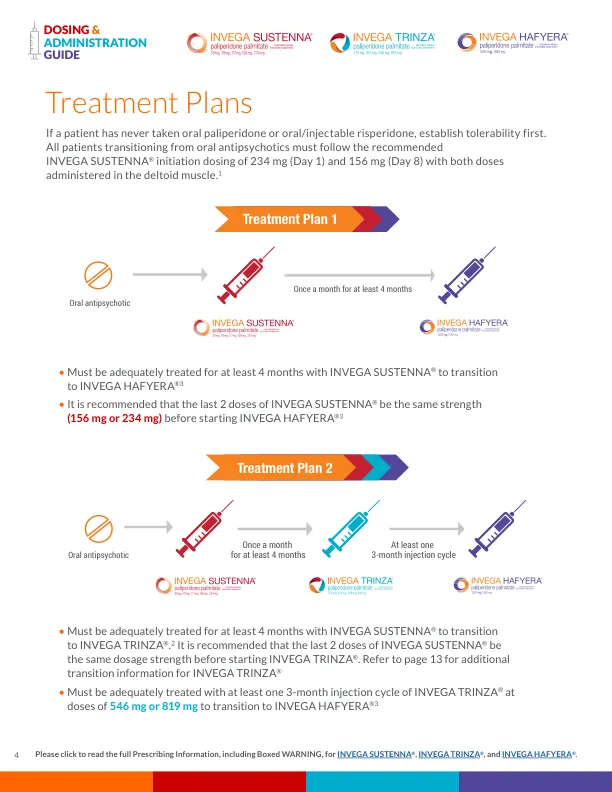
During the open-label stabilization phase of a long-term maintenance trial for INVEGA TRINZA ® for the treatment of schizophrenia, enrolled patients treated with RISPERDAL CONSTA ® long-acting injection were switched to INVEGA SUSTENNA ® in place of the next scheduled injection at a dose determined by the conversion guide. 2 It is important to note that the INVEGA SUSTENNA ® conversion dose may not reflect the eventual stabilization dose that was achieved during the remainder of the open-label transition phase.
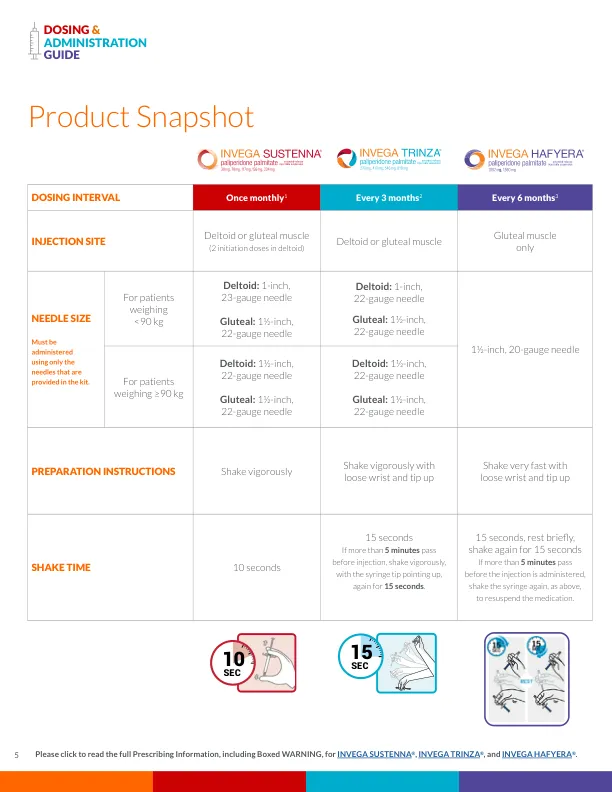
DOSING & ADMINISTRATION GUIDE
主要关键词