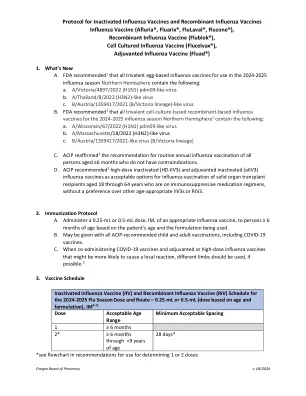
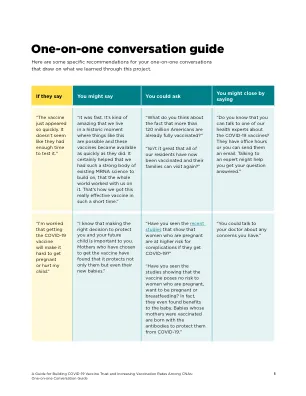
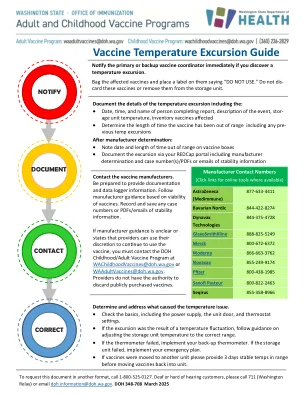
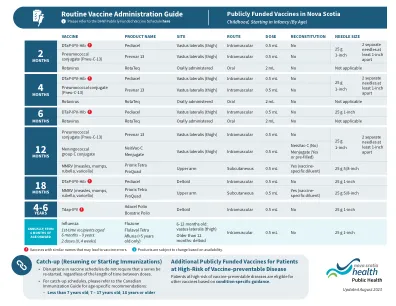
4.1。QUALITY ........................................................................................................... 4 4.1.1.生产/父母生物的底物.................................................................................................................................... 4 4.1.2。Genetic material used in the construction of the vector ......................................... 4 4.1.3 Vectors ........................................................................................................... 5 4.1.4 Sequences to be inserted ................................................................................... 5 4.1.5 Characteristics of the final vector vaccine ................................................................................................................ 5
实时重组矢量疫苗的指南
主要关键词