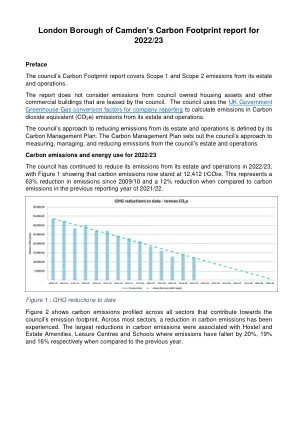
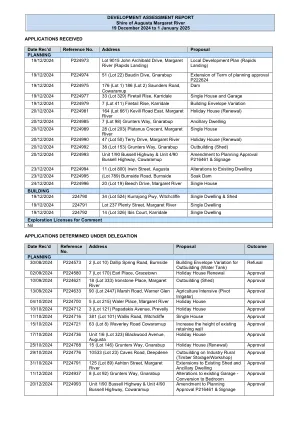
3.1 Estimated exposure .............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................. Post-registration exposure (commercial use) ....................................................................................................................................................................................................................................................................................... 关键临床研究功效的结果..................................................................................................................................................................................................................................................................................................................................................................................................................................................... RESULTS OF POST CONCERCIALIZATION EFFICIENCY ....................................................................................................................................................... KTE-C19-110/NCT01166009 ............................................................................................................................................... KT-EU-471-0117 ......................................................................... 15 3.3.3. YES020-011 ........................................................................................................163.1 Estimated exposure ..............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................Post-registration exposure (commercial use) ....................................................................................................................................................................................................................................................................................... 关键临床研究功效的结果..................................................................................................................................................................................................................................................................................................................................................................................................................................................... RESULTS OF POST CONCERCIALIZATION EFFICIENCY ....................................................................................................................................................... KTE-C19-110/NCT01166009 ............................................................................................................................................... KT-EU-471-0117 ......................................................................... 15 3.3.3. YES020-011 ........................................................................................................16Post-registration exposure (commercial use) .......................................................................................................................................................................................................................................................................................关键临床研究功效的结果.....................................................................................................................................................................................................................................................................................................................................................................................................................................................RESULTS OF POST CONCERCIALIZATION EFFICIENCY ....................................................................................................................................................... KTE-C19-110/NCT01166009 ............................................................................................................................................... KT-EU-471-0117 ......................................................................... 15 3.3.3. YES020-011 ........................................................................................................16RESULTS OF POST CONCERCIALIZATION EFFICIENCY .......................................................................................................................................................KTE-C19-110/NCT01166009 ............................................................................................................................................... KT-EU-471-0117 ......................................................................... 15 3.3.3. YES020-011 ........................................................................................................16KTE-C19-110/NCT01166009 ...............................................................................................................................................KT-EU-471-0117 ......................................................................... 15 3.3.3. YES020-011 ........................................................................................................16KT-EU-471-0117 ......................................................................... 15 3.3.3.YES020-011 ........................................................................................................16
记录监控的定期报告...
主要关键词