机构名称:
¥ 2.0
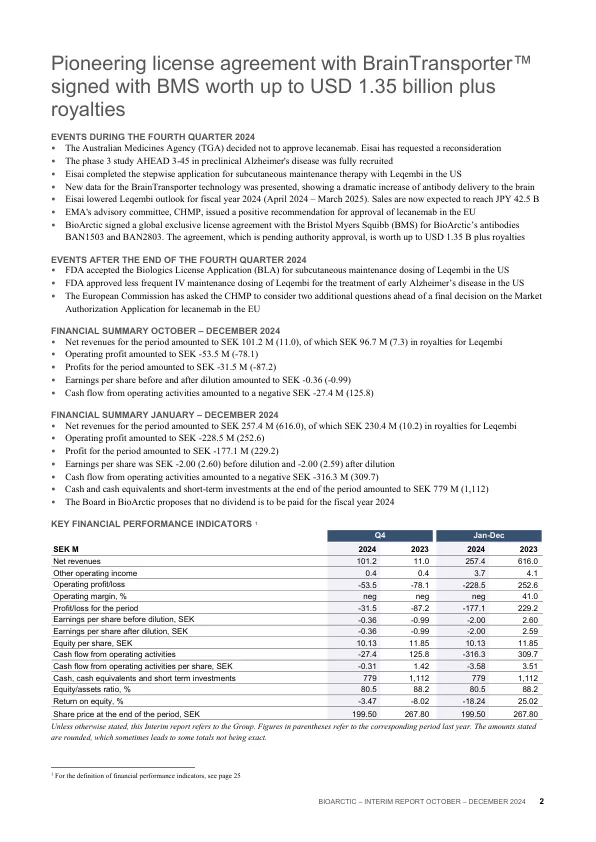
2024年第四季度的活动•澳大利亚药品局(TGA)决定不批准李卡纳姆布。Eisai has requested a reconsideration • The phase 3 study AHEAD 3-45 in preclinical Alzheimer's disease was fully recruited • Eisai completed the stepwise application for subcutaneous maintenance therapy with Leqembi in the US • New data for the BrainTransporter technology was presented, showing a dramatic increase of antibody delivery to the brain • Eisai lowered Leqembi outlook for fiscal year 2024年(2024年4月 - 2025年3月)。销售预计将达到JPY 42.5 B•EMA的咨询委员会CHMP发出了积极的建议,以批准欧盟的LeCanemab•Bioarctic与Bristol Myers Squibb(BMS)签署了一项全球独家许可协议(BMS),以提供生物极低的抗体的BAN1503和BAN2803。该协议正在等待批准,值得超过1.35 B加版本
报告-Bioarctic
主要关键词