XiaoMi-AI文件搜索系统
World File Search System经颅磁刺激Herceptin Hylecta™(Trastuzumab和Hyaluronidase- oysk)
前面的信息旨在用于非医疗保险确定。Medicare Benefit Policy手册中概述了门诊病人的医疗保险(B部分)药物(Pub。100-2),第15章,第50条药物和生物学。 此外,可能存在国家承保范围确定(NCD)和/或本地覆盖范围确定(LCD),并且在适用的情况下需要遵守这些政策。 本地覆盖范围文章(LCA)也可能出于索赔付款目的而存在,或阐明B部分根据可能自我管理的药物的福利资格。 以下链接可用于搜索NCD,LCD或LCA文档:https://www.cms.gov/medicare-coverage--database-database/search.aspx。 可以根据健康计划的酌情决定其他指示,包括任何前面的信息。100-2),第15章,第50条药物和生物学。此外,可能存在国家承保范围确定(NCD)和/或本地覆盖范围确定(LCD),并且在适用的情况下需要遵守这些政策。本地覆盖范围文章(LCA)也可能出于索赔付款目的而存在,或阐明B部分根据可能自我管理的药物的福利资格。以下链接可用于搜索NCD,LCD或LCA文档:https://www.cms.gov/medicare-coverage--database-database/search.aspx。可以根据健康计划的酌情决定其他指示,包括任何前面的信息。
Septins 破坏可控制肿瘤生长并增强赫赛汀的疗效
Septins disruption controls tumor growth and enhances efficacy of Herceptin 1 2 Rakesh K Singh* 1 , Kyu Kwang Kim 1 , Negar Khazan 1 , Rachael B. Rowswell-Turner 1 , Christian 3 Laggner 3 , Aaron Jones 1 , Priyanka Srivastava 1 , Virginia Hovanesian 4 , Liz Lamere 1 , Thomas 4 Conley 1 , Ravina Pandita 1 , Cameron Baker 5 , Jason R Myers 5 , Elizabeth Pritchett 5 , Awada Ahmad 1 , 5 Luis Ruffolo 2 , Katherine Jackson 2 , Scott A. Gerber 2 , John Ashton 5 , Michael T. Milano 6 , David 6 Linehan 2 , Richard G Moore 1 7 8 1 Wilmot Cancer Institute, Division of Gynecologic Oncology, Department of Obstetrics and 9 Gynecology, University of Rochester Medical Center, Rochester, NY, USA. 10 2 Department of Surgery, Microbiology and Immunology; Department of Radiation Oncology and 11 Center for Tumor Immunology Research, University of Rochester Medical Center, Rochester, NY, 12 USA. 13 3 Atomwise Inc, San Francisco, CA, USA. 14 4 Rhode Island Hospital, Providence, RI, USA. 15 5 Genomics Research Center, Wilmot Cancer Center, University of Rochester Medical Center, NY, 16 USA. 17 6 Department of Radiation Oncology, University of Rochester, NY, USA. 18 19 20 * Corresponding author: 21 Rakesh_Singh@URMC.Rochester.Edu 22 Telephone (office): 585-276-6281. Fax: 585-276-2576 23 24 Abstract 25 Septin expressions are altered in cancer cells and exhibit poor prognoses in malignancies. As the 26 first approach to develop a septin filament targeting agent, we optimized the structure of 27 Forchlorfenuron (FCF), a known plant cytokinin to generate UR214-9, which contrary to FCF, 28 causes septin-2/9 filamental structural catastrophe in cancer cells without altering cellular septin 29 protein levels. In-silico docking using septin-2/septin-2 dimer complex showed that UR214-9 30 displaced the guanine carbonyl oxygen from the GDP binding domain and showed increased 31 binding energy than FCF(-8.59vs-7.21). UR214-9 reduced cancer cell growth, downregulated 32 HER2/STAT-3 axis and controlled growth of HER2+ pancreatic, breast and ovarian cancer 33 xenografts in NSG mice and enhanced response of Herceptin against HER2+breast cancer 34 xenograft. Transcriptome analysis of UR214-9 exposed cells demonstrated significant 35 perturbation of <20 genes compared to afatinib which impacted >1200 genes in JIMT-1 breast 36 cancer cells indicating target specificity and non-transcriptional functions of UR214-9. In summary, 37 disrupting septins via UR214-9 is a new approach to control the growth of HER2+ malignancies. 38 39 Introduction 40 41 Septins are a family of GTP-binding cytoskeletal proteins that participate in cytokinesis, 42 cell migration, chromosomal dynamics and protein secretion. Septins hetero-oligomerize to 43 generate scaffolding filaments, bundles, and rings within cells 1-11 . Additionally, septins are a 44 critical cytoskeletal component that regulate the function of tubulin and actin. Altered septin 45
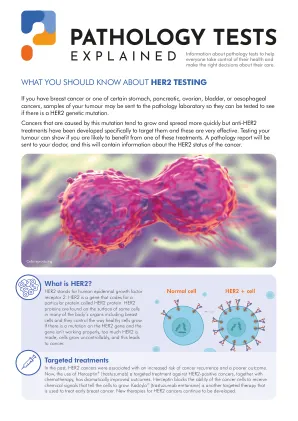
您应该了解HER2测试
过去的有针对性治疗方法,HER2癌症与癌症复发的风险增加和结果较差有关。现在,使用Herceptin®(Trastuzumab)对HER2阳性癌症的靶向疗法以及化学疗法的结合已大大改善了结果。赫斯汀蛋白阻止了癌细胞接收能够告诉细胞生长的化学信号的能力。kadcyla®(曲妥珠单抗Emtansine)是另一种用于治疗早期乳腺癌的靶向疗法。HER2癌症的新疗法继续开发。
MR_CHMP建议欧盟批准Phesgo_en
•phesgo在短短几分钟内,在皮肤下提供更快,更少的侵入性提供护理标准治疗,而静脉输注1,2,3•皮下给药的偏爱是患者,医生和医疗保健提供者的首选,并且可以与医院的时间和成本相关联,这是•第一个概述的人群•同时是•近期•同时,这是两次comploy•hiptib y him in tros in comploy intib•himbon tros in comploy intib y 5,5,5,6• 2020年11月13日,由单一皮下注射巴塞尔(Roche)(六:RO,ROG; OTCQX:ROG; OTCQX:RHHBY)今天宣布,欧洲药品局(EMA)人类使用药物委员会(CHMP)推荐了Perjeta®的固定剂量组合(perjetaiumabimabimabimabimabimabimabimabimab)透明质酸酶,由皮下(SC;在皮肤下)和静脉内(IV)化学疗法结合使用,以治疗早期和转移性HER2阳性乳腺癌。基于这一建议,预计欧盟委员会在不久的将来有望获得有关Phesgo的最终决定。“我们致力于改变乳腺癌患者生活的承诺远远超出了改善疗效结果,”罗氏首席医学官兼全球产品开发负责人Levi Garraway博士说。“今天的建议是重新定义欧洲患有HER2阳性乳腺癌患者的护理标准的又一重要一步,它可能会提供一种更快,更侵入性的方式来接受Perjeta和Herceptin接受治疗。” Phesgo的SC给药大约需要八分钟的初始加载剂量,每个随后的维持剂量大约需要五分钟。1与使用标准IV配方输注perjeta和Herceptin的载荷剂量的大约150分钟相比,随后对两种药物进行维护输注60-150分钟。2,3 CHMP的建议是基于III期Federica研究的结果,该研究表明,与两种药物相比,使用Phesgo的治疗在血液中产生了非内部perjeta和Herceptin。对化学疗法的Phesgo的安全性与Perjeta Plus Herceptin和化学疗法的静脉注射疗法相当,并且没有发现新的安全信号,包括心脏毒性的有意义差异。两个臂中最常见的不良事件是脱发,恶心,腹泻和贫血。1,7
专科医疗福利药品清单
Antineoplastic Agents Abraxane, Adcetris, Adstiladrin, Alimta, Aliqopa, Alymsys, Anktiva, Arzerra, Asparlas, Avastin, Avzivi, Azedra, Beleodaq, Belrapzo, Bendeca, Besponsa, Blenrep, Blincyto, Columvi, Cyramza, Danxel, Darzale, Elzale, Elzala, Eprozahere rexfio, Elzonris, Empliciti, Enhertu, Epkinly, Erbitux, Erwinaze, Faslodex, Firmagon, Folotyn, Gazyva, Halaven, Hepzato Kit, Herceptin, Herceptin Hylecta, Hercessi, Herzuma, Imdelltra, Imjudo, Imlygic, Intron-A, Istomy, Jela, Jel, Katjin, Khapzory, Kimmtrak, Kyprolis, Lunsumio, Margenza, Monjuvi, Mvasi, Mylotarg, Ogivri, Oncaspar, Ontruzant, Opdualag, Padcev, Pemfexy, Pemrydi RTU, Perjeta, Phesgo, Pluvicto, Polivy, Portrazza, Poteligeo, Provence, Rioxila, Hyunce, Rtu Rix vant, Rylaze, Sarclisa, Synribo, Talvey, Tecvayli, Temodar, Tivdak, Trazimera, Treanda, Trodelvy, Truxima, Vegzelma, Vivimusta, Vyxeos, Xofigo, Yervoy, Zepzelca, Zevalin, Zirabev, Zynlonta
需要预先认证的服务
Alimta, Alymsys (except for ophthalmological conditions), Anktiva, Avastin (except for ophthalmological conditions) 5 , Avzivi, Azedra 2 , Blincyto, Columvi, Cyramza, Darzalex, Darzalex Faspro, datapotamab deruxtecan 6 , Elaux, Herbi, Erbithere, Erbithere, 5 in Hylecta, Hercessi, Herzuma, Imjudo, Kadcyla, Kimmtrak, Kyprolis, Margenza, Monjuvi, odronextamab 6 , Ogivri, Ontruzant, Opdualag, Padcev, patritumab deruxtecan 6 , Pemfexy, Perjeta, Phesgo, Pluvicto, Polio, Rio, Rio, Provence, 5 , Rituxan Hycela, Ruxience, Rybrevant, Rylaze, Sarclisa, Taclantis, Talvey, Tecvayli, Tivdak, trastuzumab duocarmazine 6 , Trodelvy, Vegzelma (except for ophthalmological conditions), Xofigo 2 , Yervoy, zanidat 6, Zepzelca, Zepzelca , Zynlonta • Anti-PD-1/PD-L1 human monoclonal antibodies: 4
专科医疗福利药品清单
Antineoplastic agents Abraxane, Adcetris, Adstiladrin, Alimta, Aliqopa, Alymsys, Anktiva, Arzerra, Asparlas, Avastin, Avzivi, Azedra, Beleodac, Belrapzo, Bendeca, Besponsa, Blenrep, Blincyto, Columvy, Cyram, Darzale, Darzale, Esa, Esa, Fax antibiotic, Elrexfio, Elzonris, Empliciti, Enhertu, Epkinly, Erbitux, Erwinaze, Faslodex, Firmagon, Folotyn, Gazyva, Halaven, Hepzato Kit, Herceptin, Herceptin Hylecta, Hercessi, Herzuma, Imdelltra, Imjudo, Imlygic, Instoron-A, Jelato, Jelato, Jena, Kanjinti, Khapzory, Kimmtrak, Kyprolis, Lunsumio, Margenza, Monjuvi, Mvasi, Mylotarg, Ogivri, Oncaspar, Ontruzant, Opdualag, Padcev, Pemfexy, Pemrydi RTU, Perjeta, Phesgo, Pluvicto, Polivy, Portrazza, Potelige, Provence, Hyundai, Hyundai, Rioxila , Rybrevant, Rylaze, Sarclisa, Synribo, Talvey, Tecvayli, Temodar, Tivdak, Trazimera, Treanda, Trodelvy, Truxima, Vegzelma, Vivimusta, Vyxeos, Xofigo, Yervoy, Zepzelca, Zevalin, Zirabev, Zynlonta >
需要预先认证的服务
(眼科条件除外)•Avzivi®(bevacizumab-tnjn)•Azedra®‡(Iobenguane I-131)•blincyto®(blinatumomab)•gulofitamab(glofitamab) otamab deruxtecan*•elahere®(mirvetuximab soravtansine-gynx)•elrexfio™(Elranatamab-bcmm)•Enhertu(Fam-trastuzumab-deruxtecan-nxki) Hercessi™(trastuzumab-strf)•Herzuma®(trastuzumab-pkrb)•imjudo®(tremelimumab)•kadcyla®(ado-trastuzumab emtansinel) juvi®(tafasitamab-cxix)•odRonextamab*•ogivri™(trastuzumab-dkst)•ontruzant®(trastuzumab-dttb)•oplimab-br-br-bdb)v™ ®(pertuzumab)•Phesgo™(pertuzumab/trastuzumab/hyaluronidase-zzxf)•pluvicto™(leute vidotin-17)(polatuzumab vedotin vedotin-piiq) ®†(Rituximab)•Rituxan Hycela™(利妥昔单抗/透明质酸酶Humane)•Rituximab-vavant)
年度报告2023
在未满足的医疗需求方面,也许没有比癌症更合适的例子,这是日本死亡1的主要原因。chugai已开发并推出了连续的一系列高度创新的药物,包括从罗氏(Roche)许可的产品,这些产品有助于改善预后治疗各种癌症。以HER2阳性乳腺癌和Alk-阳性肺癌为例,这两者以前很难治疗。 chugai在对抗这些癌症作战方面的贡献包括抗HER2抗体Herceptin,Perjeta和Kadcyla,以及分子靶向药物(例如内部产品Alecensa)。 通过中型分子和其他方式的演变,Chugai将大胆地应对以前未解决的艰难靶标的挑战,希望增加可用于克服未满足医疗需求的治疗药物。以HER2阳性乳腺癌和Alk-阳性肺癌为例,这两者以前很难治疗。chugai在对抗这些癌症作战方面的贡献包括抗HER2抗体Herceptin,Perjeta和Kadcyla,以及分子靶向药物(例如内部产品Alecensa)。通过中型分子和其他方式的演变,Chugai将大胆地应对以前未解决的艰难靶标的挑战,希望增加可用于克服未满足医疗需求的治疗药物。