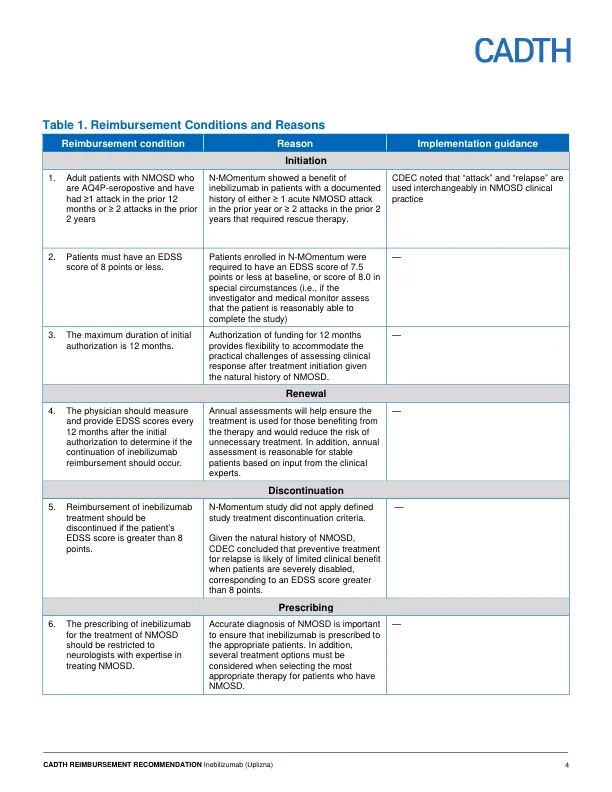
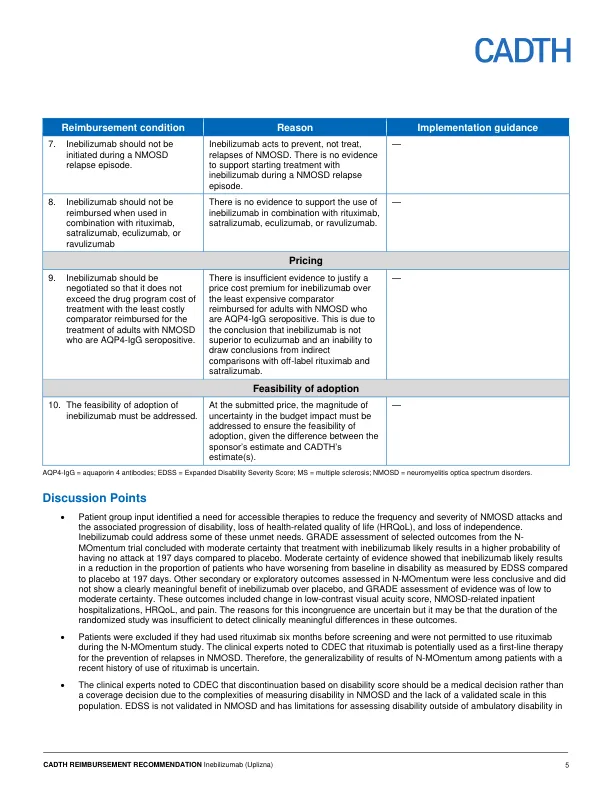
One double-blind, phase 2/3, randomized controlled trial (RCT) (N-MOmentum, N = 230) in patients with NMOSD demonstrated that, among AQP4 seropositive patients (N = 213) treatment with inebilizumab likely results in a clinically meaningful increase in time to first relapse and a reduction in the proportion of patients with worsening in Expanded Disability Status Scale (EDSS) compared to treatment with placebo after 197 days of treatment. Among AQP4 seropositive patients, the hazard ratio (HR) for time to first Adjudication Committee (AC)–determined NMOSD attack was 0.28 (95% confidence interval [CI]: 0.12 to 0.42, P < 0.0001) and at day 197, 87.6% of patients in the inebilizumab group were attack-free compared to 56.6% in the placebo group, which equated to a 77.3% reduction in the risk of AC-determined NMOSD attack. The CADTH review of the sponsor's submitted indirect treatment comparisons concluded that inebilizumab did not offer a benefit relative to eculizumab, and that imprecision in the estimated HR prevented a definitive conclusion from being reached in the comparison with satralizumab.
CADTH Reimbursement Recommendation
主要关键词