机构名称:
¥ 2.0
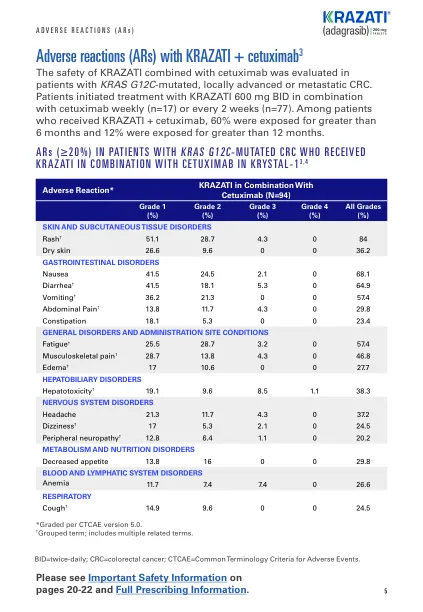
• ARs requiring dosage interruption in ≥2.0% of patients who received KRAZATI included diarrhea, nausea, vomiting, abdominal pain, dizziness, headache, pneumonia, alanine aminotransferase increased, aspartate aminotransferase increased, dyspnea, fatigue, pleural effusion, rash, anemia, electrocardiogram QT延长,血液胆红素增加,血肌酐增加,食欲降低,脱水,出血,低磁性血症,脂肪酶增加,肌肉无力,肌肉无力,肌肉骨骼疼痛和去虫剂量降低,导致35%的患者
Krazati治疗管理指南