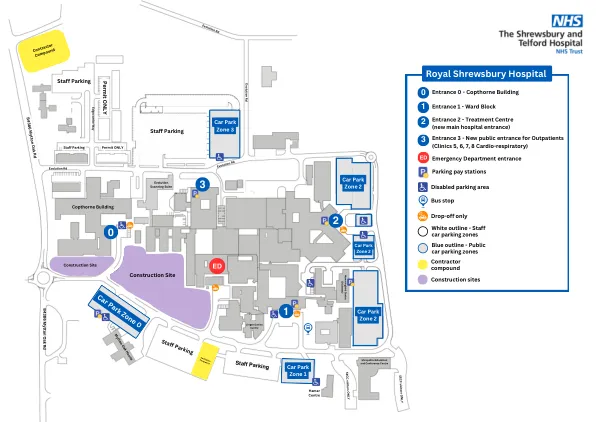
2.1。问题说明...................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................Disease or condition....................................................................................... 11 2.1.2.流行病学和风险因素.................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................Aetiology and pathogenesis ............................................................................. 11 2.1.4.Clinical presentation and diagnosis ................................................................... 12 2.1.5.管理...................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................质量方面.....................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................Introduction ................................................................................................. 14 2.2.2.Active Substance ........................................................................................... 15 2.2.3.制成的药品............................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................. 22 2.2.4。Discussion on chemical, pharmaceutical and biological aspects ............................. 31 2.2.5.关于化学,药物和生物学方面的结论.............................................. 35 2.2.6。未来质量发展的建议........................................................................................................................................................................................................................................................................................................................................................................................................................................................................... 39 2.3。非临床方面................................................................................................................................................................................................................................................................................................................................................................................................................................................................... 41 2.3.1。Pharmacology ............................................................................................... 41 2.3.2.药代动力学........................................................................................................................................................................................................................................................................................................................................................... 45 2.3.3。 Toxicology .................................................................................................... 48 2.3.4. Ecotoxicity/environmental risk assessment ........................................................ 51药代动力学........................................................................................................................................................................................................................................................................................................................................................... 45 2.3.3。Toxicology .................................................................................................... 48 2.3.4.Ecotoxicity/environmental risk assessment ........................................................ 51
comirnaty-epar-public-assessment-report_en.pdf
主要关键词