机构名称:
¥ 1.0
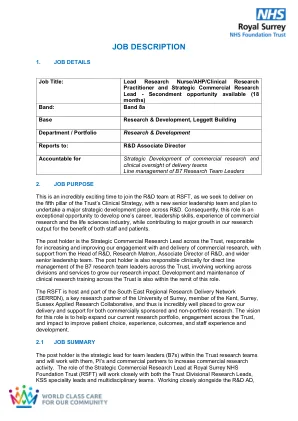
• Experience in research clinical trials administration including working knowledge of regulatory and governance requirements for clinical trials • Demonstrable experience in an administrative role working in an office environment • Excellent written and oral communication skills • Proven evidence of excellent organisational and time management skills • Ability to work unsupervised taking responsibility for own actions, including appropriate use of initiative and problem solving • Comprehensive knowledge of standard office software packages and IT skills including database entries and queries (Excel and Access) •使用机密信息(例如医疗记录或临床试验参与者信息)的经验•以服务为导向的方法,灵活,可靠和积极主动地学习不断变化的研究团队的需求•根据程序,规则和法规
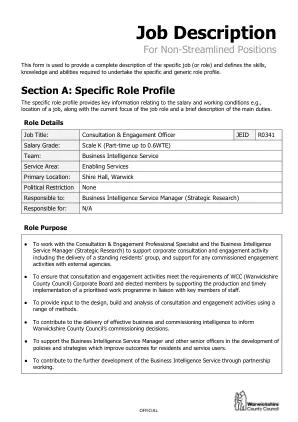
临床试验助理 - 职位描述和选择标准
主要关键词