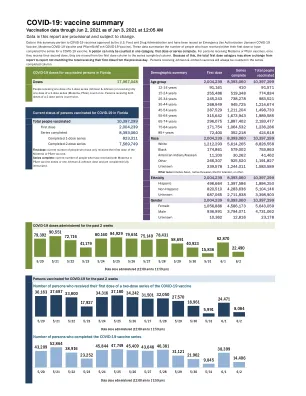
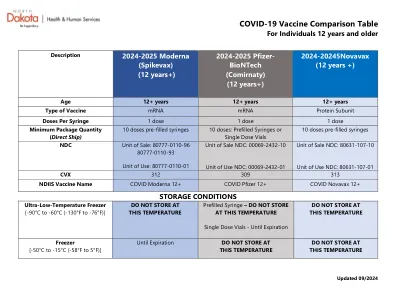
疫苗组。发病时间为第 1 剂后第 37 天(参与者未接种第 2 剂)和第 2 剂后第 3、9 和 48 天。安慰剂组未报告急性周围性面瘫(或麻痹)病例。f. 上市后确定的不良反应。g. 上市后阶段,有注射皮肤填充剂史的疫苗接种者报告面部肿胀。参考文献 1. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf 2. https://www.who.int/news/item/27-10-2021-gacvs-statement-myocarditis-pericarditis-covid-19-mrna-vaccines-updated 3. https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a 4. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#recommendations 5. USFDA Moderna COVID-19 疫苗医疗保健提供者情况说明书( https://www.fda.gov/media/144637/download) 6. USFDA 审查备忘录附录CBER 于 2021 年 11 月 18 日发布的审查备忘录,标题为“CBER 对 18 岁及以上个体在进行 COVID-19 初级免疫系列后接种 Moderna COVID-19 疫苗(0.25 毫升)加强剂量的评估” 7。https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-01-05/02-COVID-Su-508.pdf 8。https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-05-05-2022 9。https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-04-20/03-COVID-Klein-Shimabukuro-508.pdf 10。 https://www.gov.il/BlobFolder/reports/vaccine-efficacy-safety-follow-up-committee/he/files_publications_corona_29032022.pdf 11. https://www.fda.gov/media/153713/download 12. https://www.fda.gov/media/153714/download
BioNTech (BNT162b2) COVID-19 疫苗说明书
主要关键词