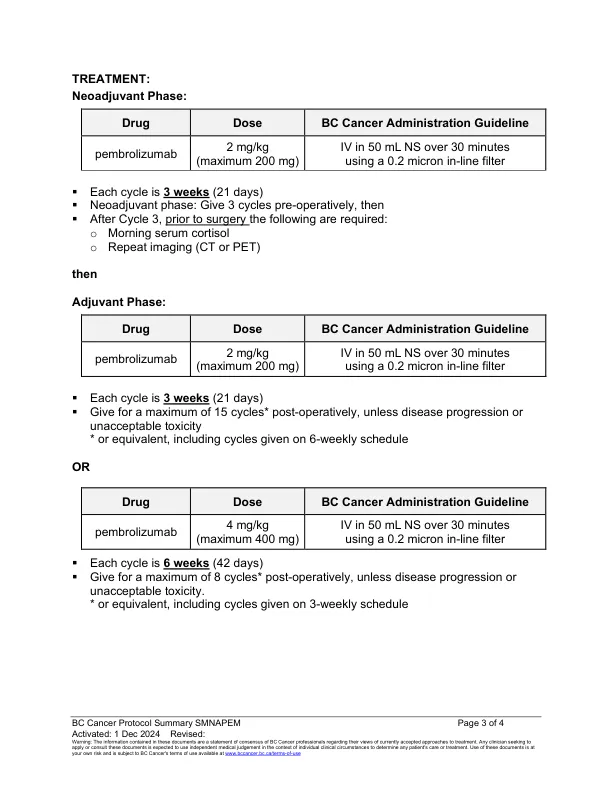
资格:患者必须具有:可切除的皮肤,丙氨酸或粘膜黑色素瘤,临床可检测到的临床可检测到的淋巴结疾病,或者在介绍时可切除的转移性疾病有限[即IIIB到IIIB或IIIIS或IIII IIIIS或IIIIS或寡聚期IV期IV期IV(M1A,M1B和M1B和M1C)的效果(M1B和M1B)的疗法,并疗法为8 th the Edition and untist and untist and Edition],并且是Edition and Edities。足够的基线血液学,肝功能和肾功能进入具有管理免疫疗法的专业知识的治疗中心介导的Pembrolizumab的毒性,pembrolizumab的毒性注释:pembrolizumab的三个周期应在术前进行术前接受(新辅助治疗阶段),除非需要进行6个周期的临床批准,否则需要进行临床批准,否则需要进行临床批准,否则需要进行临床批准。 pembrolizumab术后(辅助治疗阶段)。Do not switch to 6- weekly dosing during neoadjuvant treatment phase Patients may have subsequent checkpoint inhibitors for advanced disease if last adjuvant pembrolizumab dose was > 6 months prior EXCLUSIONS: Patients must not have: Uveal or ocular melanoma CAUTIONS: Concurrent autoimmune disease Patients with long term immunosuppressive therapy or systemic corticosteroids (requiring超过10 mg泼尼松/天或同等的)
BC癌症协议摘要 - 新辅助...