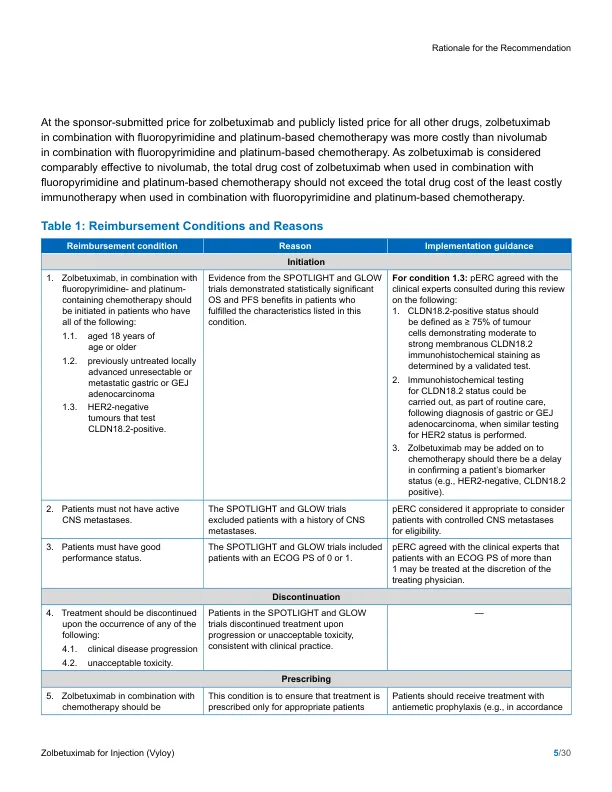
来自2个随机,双遮盖的,安慰剂对照的III期试验(Spotlight and Glow)的证据表明,Zolbetuximab添加到氟吡啶丁胺 - 和铂的化学疗法中时(改良的氟尿嘧啶加氟尿嘧啶加上白细胞素的改性,以及对OXALIPPATIN 6 [MFOLFOLFOFFOOX 6]或CAPECITIPLAT []或CAPECITIPLAT []或CAPECITIPLATIN []或CAPECALIPLATIN []患有局部晚期不可切除或转移性HER2-阴性胃癌或GEJ腺癌的患者具有CLDN18.2阳性的肿瘤患者,从而增加了临床益处。The SPOTLIGHT trial (N = 565) demonstrated that treatment with zolbetuximab in combination with mFOLFOX6 resulted in statistically significant and clinically meaningful improvements in overall survival (OS) (hazard ratio [HR] = 0.784; 95% confidence interval [CI], 0.644 to 0.954; P = 0.0075) and progression-free survival (PFS) (HR = 0.751;与安慰剂结合使用MFOLFOX6,CI,0.598至0.942;The GLOW trial (N = 507) similarly demonstrated that treatment with zolbetuximab in combination with CAPOX resulted in statistically significant and clinically important meaningful improvements in OS (HR = 0.763; 95% CI, 0.622 to 0.936; P = 0.0047) and PFS (HR = 0.687; 95% CI, 0.544 to 0.866; P = 0.0007),与安慰剂与Capox结合使用相比。在关键试验中,与单独的化学疗法相比,用化学疗法结合使用Zolbetuximab与化学疗法结合使用,与恶心,呕吐和输注相关的反应的风险增加有关。但是,PERC与临床专家一致,这些不良事件(AES)在临床实践中可能是可以管理的。
Zolbetuximab注射(Vyloy)
主要关键词