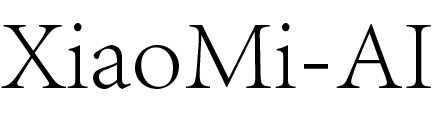机构名称:
¥ 1.0
• As of May 2, 2024 all BCR::ABL1 RT-PCR quantitative testing (major and minor) will be transitioned to the Health Canada-approved Asuragen QuantideX qPCR BCR-ABL IS kit and Minor kit respectively, due to recent lack of availability and quality issues with the previously utilized BCR::ABL1 reagents (QIAGEN Ipsogen kits).新测定法检测到与先前测定的相同的成绩单变体,并根据通过IRIS建立的国际标准(用于主要断点)报告。新的主要断点测定法具有略有改善的转录检测下限(log 4.7降低 /%为0.002%;先验套件,log 4.5降低)。新的次要断点测定法具有提高的转录检测的下限(log 4.61降低 /%为0.0025%;先验套件,log 3.6降低)。
更改CALGARY区和南部区域的BCR :: ABL1定量测试