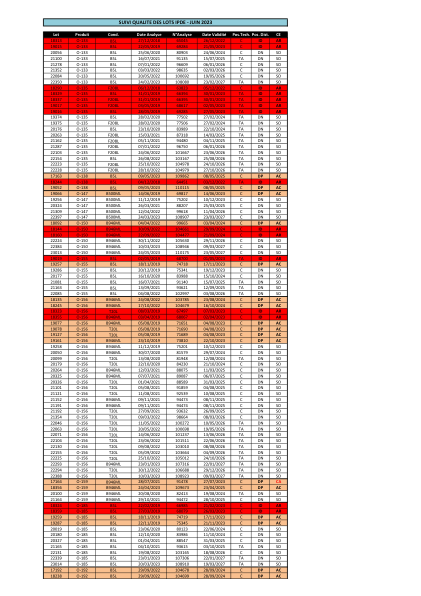
机构名称:
¥ 1.0
This submission is a safety review labeling supplement for antihemophilic factor (Recombinant), single chain (rVIII single chain) licensed in 2016 for the treatment of adults and children with hemophilia A for on demand treatment and control of bleeding episodes, routine prophylaxis to reduce frequency of bleeding episodes, and perioperative management of bleeding.申请人从研究CSL627_3001的ARM 2(PUP)提交的数据,以支持将有关PUP处理的信息添加到包装插入物中的建议。该机构已经在研究CSL627_3001 ARM 1和3的先前治疗患者(PTP)的初步批准的Afstyla批准并审查此PAS标记补充剂的疗效数据的审查是不必要的,鉴于对Afstyla
2023年6月9日临床评论-Afstyla
主要关键词