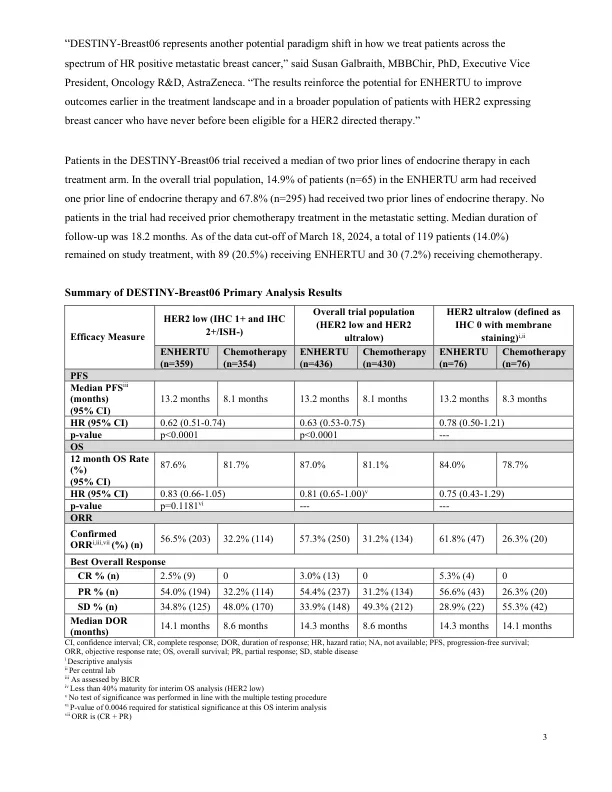
机构名称:
¥ 1.0
months of completing therapy • Unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy • Unresectable or metastatic non-small cell lung cancer (NSCLC) whose tumors have activating由FDA批准的测试检测到的HER2(ERBB2)突变,并且已经接受了先前的全身治疗,该指示根据客观响应率和响应持续时间得到加速批准。在验证性试验中,持续批准了此指示可能取决于对临床益处的验证和描述。•局部先进或转移性HER2阳性(IHC 3+或IHC 2+/ISH阳性)胃或胃食管食管连接(GEJ)腺癌,他们接受过先前的基于曲妥珠单抗的治疗方案
EnherTu®展示了中值的无进展...
主要关键词