机构名称:
¥ 1.0
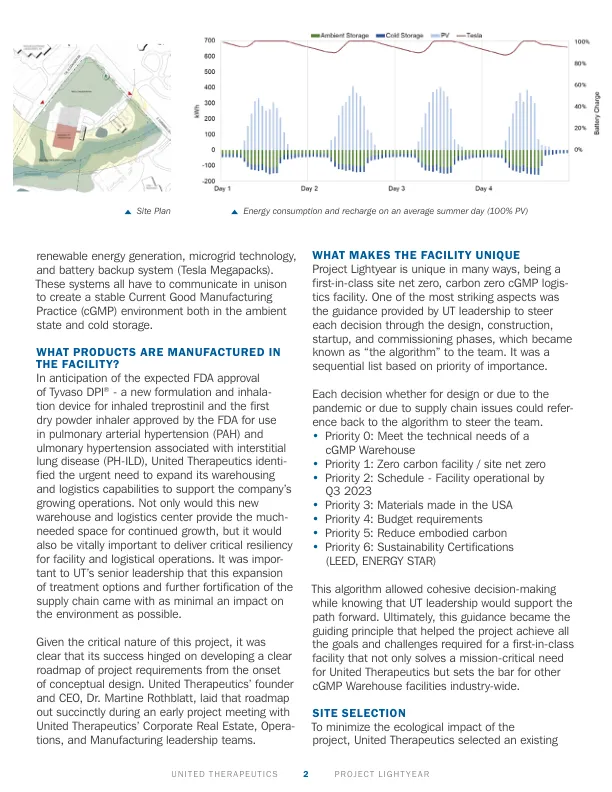
该设施中生产了哪些产品?In anticipation of the expected FDA approval of Tyvaso DPI ® - a new formulation and inhala- tion device for inhaled treprostinil and the first dry powder inhaler approved by the FDA for use in pulmonary arterial hypertension (PAH) and ulmonary hypertension associated with interstitial lung disease (PH-ILD), United Therapeutics identi- fied the urgent need to expand它的仓储和物流功能可支持公司不断增长的业务。这个新的仓库和物流中心不仅会为持续增长提供急需的空间,而且对于为设施和后勤运营提供关键的弹性至关重要。对于UT的高级领导而言,这种治疗方案的扩展和供应链的进一步强化至少对环境的影响至少。
United Therapeutics -Project Lightyear
主要关键词