机构名称:
¥ 2.0
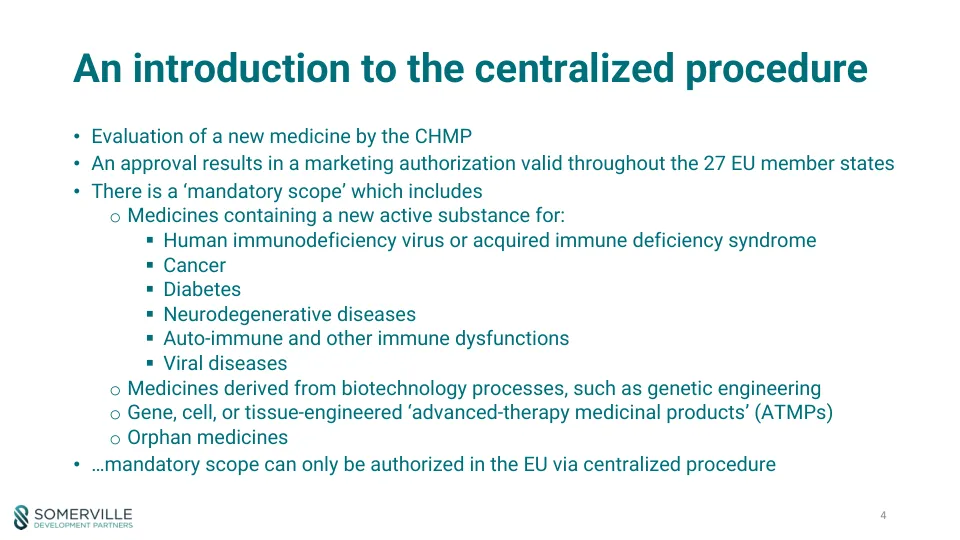
§ Human immunodeficiency virus or acquired immune deficiency syndrome § Cancer § Diabetes § Neurodegenerative diseases § Auto-immune and other immune dysfunctions § Viral diseases o Medicines derived from biotechnology processes, such as genetic engineering o Gene, cell, or tissue-engineered ‘advanced-therapy medicinal products' (ATMPs) o Orphan药品•…强制性范围只能通过集中程序在欧盟授权
'在欧盟制定有效的科学建议策略'
主要关键词