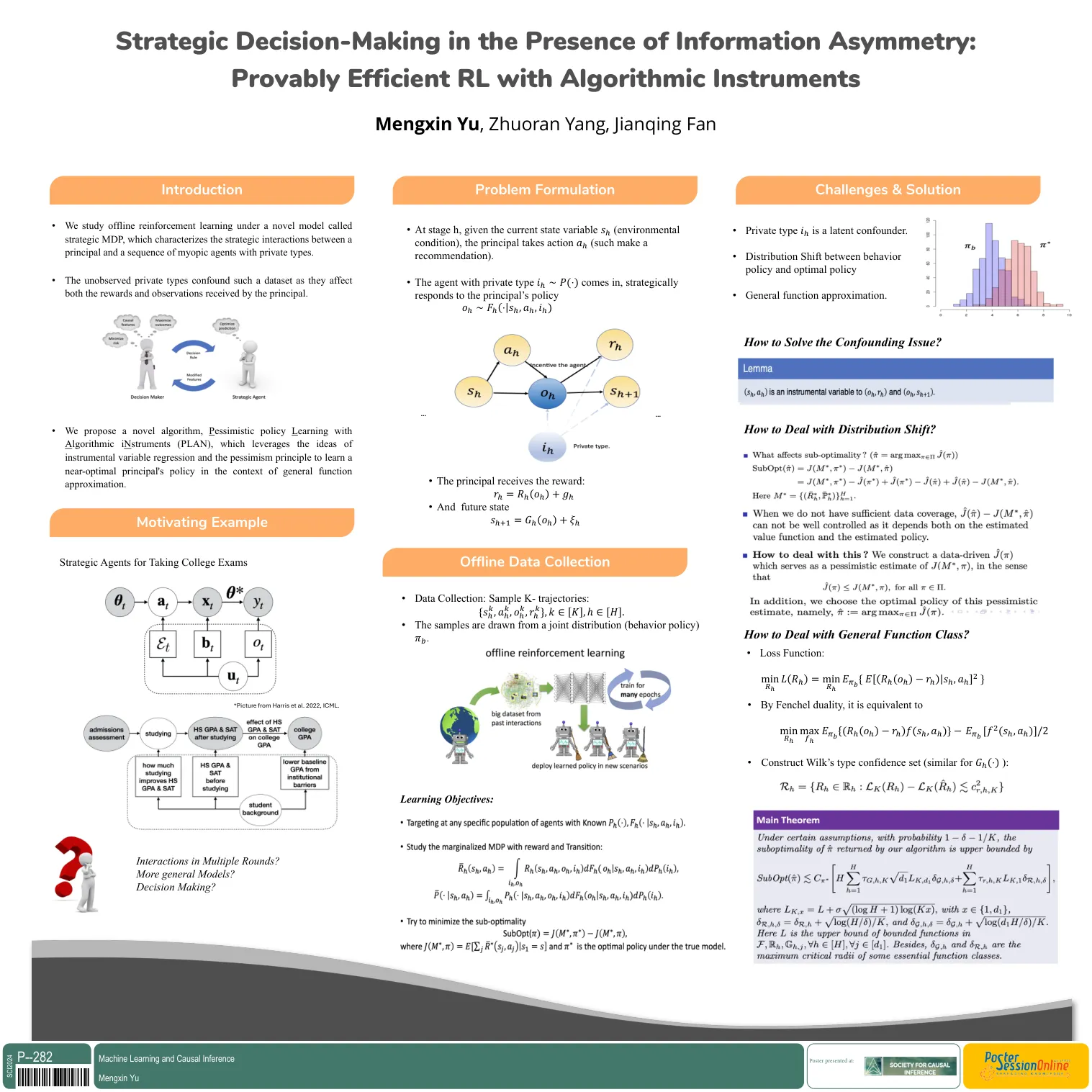
诱导的所需基因表达一直是揭示基因功能和调节合成生物学和治疗应用的细胞活性的重要策略。Apart from ectopically expressing additional copies of a gene by introducing their open reading frames (ORFs), methods to arti fi cially activate endogenous copies of genes have been explored, including transcription activating factors tethered to zinc fi nger proteins ( Beerli et al., 2000 ) and transcription activator-like effectors (TALE) ( Miller et al., 2011 ; Zhang et al., 2011 ; Maeder等人,2013b; Perez-Pinera等,2013b)。Originally discovered as a virus-resistance mechanism from bacteria ( Barrangou et al., 2007 ), the CRISPR-Cas system has provided ef fi cient, precise, and scalable ways to modulate expression of genes, and has been successfully adopted for targeted gene activation ( Mali et al., 2013 ; Perez-Pinera et al., 2013a ; Maeder et al., 2013a ; Cheng et al., 2013年,Tanenbaum等人,2014年;为了使用CRISPR-CAS9实现基因激活,创建了催化失活的Cas9(DCAS9),以与特定的基因组区域结合而没有能力创建双链突破(Jinek et al。,2012; Gasiunas et al。,2012; Qi et al。,2013; Qi et al。,2013; Konermann et; Konermann et al an al an eal; konermann et al。,2013; a e e,2013; i。赋予DCAS9具有诱导基因表达的能力,已经探索了不同的转录激活域的基因激活强度(图1A)。第一代CRISPRA的灵感来自锌纤维和基于故事的方法,并使用了包括VP64或P65在内的单个激活域。vp64由VP16的四个副本组成,该副本是源自单纯疱疹病毒的转录激活因子。p65是NF-κB复合物的一部分,负责免疫信号传导中的转录激活。第二代CRISPRA系统制定了不同的策略来招募不同的激活剂的多个副本,包括用于招募10或24份VP64副本的Suntag阵列到给定的基因座,VP64,P65和RTA(VPR)的串联融合到DCAS9,以及
转录激活的神经元细胞类型工程
主要关键词