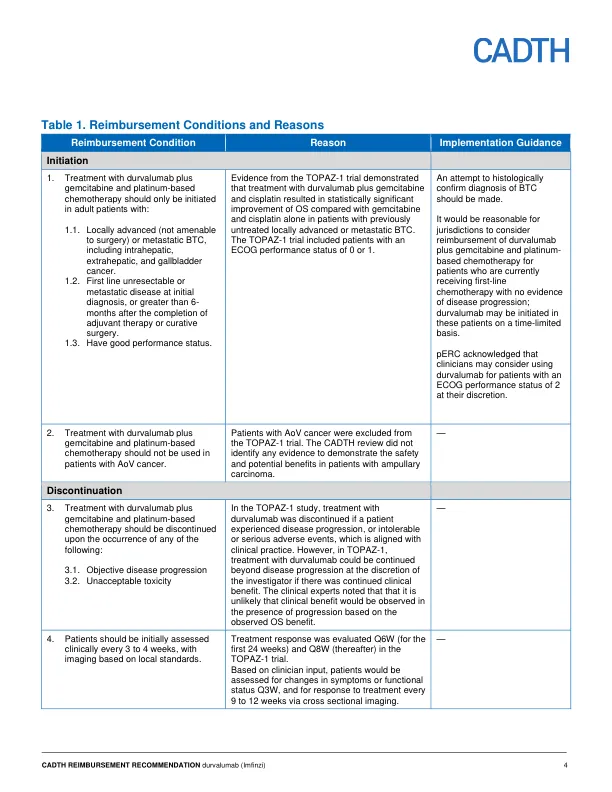
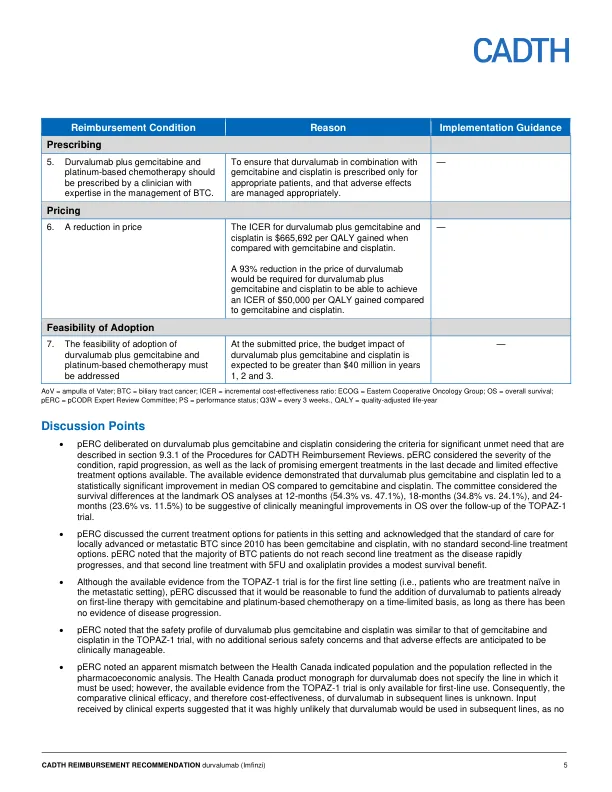
机构名称:
¥ 1.0
One international, double-blind, randomized, phase III study (TOPAZ-1) consisting of previously untreated adult patients with locally advanced or metastatic BTC demonstrated that treatment with durvalumab plus gemcitabine and cisplatin resulted in a statistically significant overall survival (OS) advantage compared to placebo plus gemcitabine and cisplatin (median OS, 12.9 months [95% CI, 11.6 to 14.1个月]与11.3个月[95%CI,10.1至12.5个月],HR,0.76 [95%CI,0.64至0。91])。在地标为12-(54.3%对47.1%),18-(34.8%对24.1%)和24.1%)和24个月(23.6%vs. 11.5%)的OS的其他分析支持了Durvalumab Plus Plus Gemcitabine和gemcitabine和cisplatin所证明的生存优势。durvalumab也与无进展生存率的改善有关([PFS] HR,0.75 [95%CI,0.63至0.89]),并且没有其他严重的安全问题,可管理的毒性概况。TOPAZ-1试验的结果也表明HRQOL没有损害。
Cadth报销建议
主要关键词