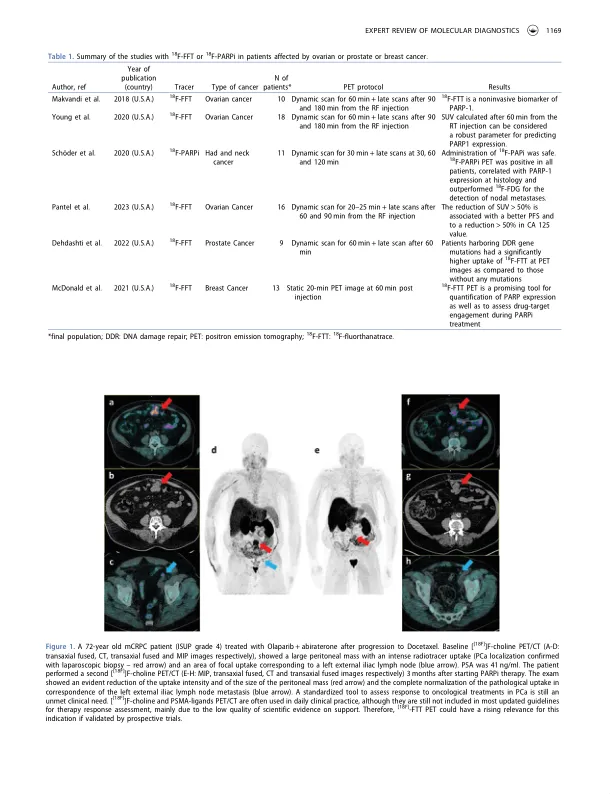
摘要简介:多-ADP-核糖 - 聚合酶抑制剂(PARPI)在肿瘤学实践中成功实施了所谓的“合成致死性”的过程。但是,并非所有患者都对PARPI做出反应,并且对适用于PARPI治疗期间患者选择和监测的非侵入性生物标志物的需求未满足。Areas covered: The first clinical applications of molecular imaging with positron emission tomography/ computed tomography (PET/CT) with [18F]-FluorThanatrace ([18F]-FTT) and [18F]-PARPi, highly effective PARP-ligands, in patients with several malignancies (head and neck, ovarian, prostate, and breast cancer) are covered, with a particular focus on its potential for预处理选择和随访。Expert opinion: By a search made on the most common database, such as PubMed and Google Scholar in a period from January 2010 and 2023, first clinical evidence suggests that PET/CT with [18F]-FTT and [18F]-PARPi might represent a reliable tool for in vivo imaging and quantification of PARP-1 expression in ovarian, prostate, breast, head, and neck cancer, supporting their potential usefulness for patient selection before parpi-therapies。此外,在治疗开始后已经注册了[18F] -FTT摄取的降低,并且似乎与基于PARPI的方案后的患者结局相关。需要进一步的研究来更好地解决这些临床环境中PARPI-Radiolabele pet成像的价值,尤其是因为它涉及技术特征,例如最佳扫描方式(动态与静态)和时机。
癌症中PARP的分子成像:最先进的
主要关键词